orbital diagram for boron
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. The s-orbital can have a maximum of two electrons.
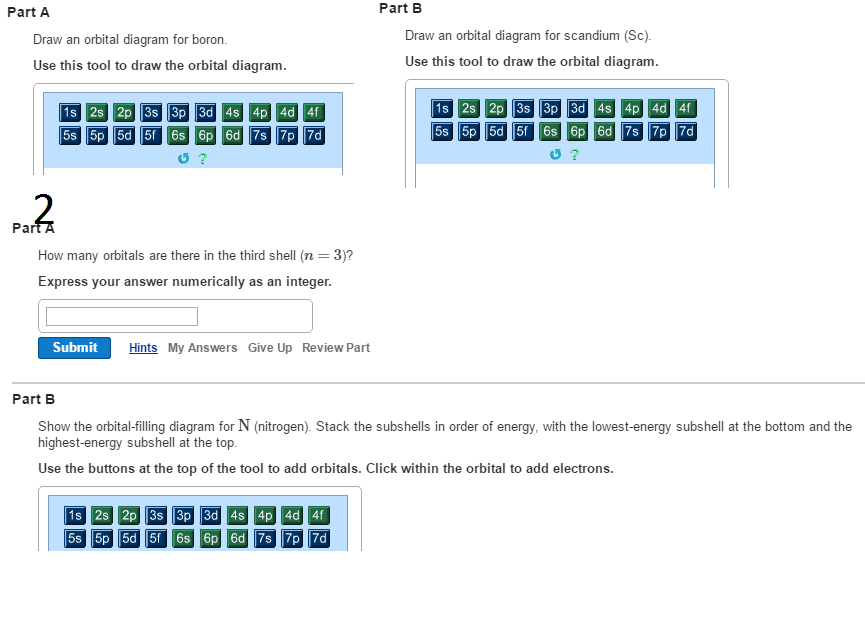
Solved Draw An Orbital Diagram For Boron Use This Tool To Chegg Com
The boron group across to the noble gases all have their outer electrons in p orbitals.
. Shapes of atomic orbital. The main group elements with an odd atomic number include the alkali metal family boron family nitrogen family and halogen family. Electronic configuration Of Elements.
This isnt a mistake but an effect of converting my original diagram into a lower quality gif image for efficient web use. The caesium Cs is a main group element located in period 6 of the periodic table. Given below is the image of the molecular orbital diagram of H3O and also that of H2O for reference.
For many molecules the sharing of electrons allows each atom to attain the. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. 1s is the closest and lowest energy orbital to the nucleus.
The term was coined and named after Serbian geophysicist and astronomer Milutin MilankovićIn the 1920s he hypothesized that variations in eccentricity axial tilt and precession combined to result in cyclical variations in the intra-annual and latitudinal. We already know that the p-subshell has three orbitals. Khuyagbaatar and others states the superheavy element with atomic number Z 117 ununseptium was produced as an evaporation residue in the 48 Ca and 249 Bk fusion reaction at the gas-filled recoil separator TASCA at GSI Darmstadt Germany.
Cell populations have been found that promote immunosuppressive regulatory T cells of the immune system in the gut. Which has been discussed in detail above. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
Carbon Bohr Model Diagram Steps to Draw. Therefore the next two electrons enter the 2s orbital. The radioactive decay of evaporation residues and their α-decay products.
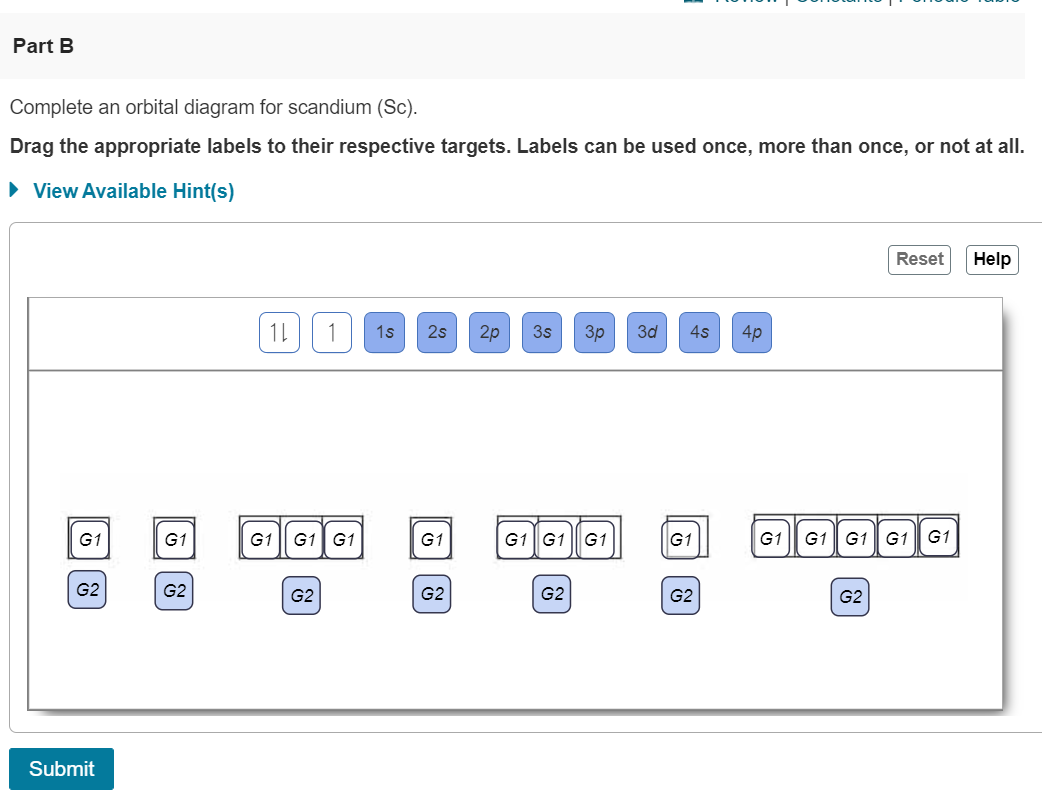
Term symbols with LS coupling. The orbital diagram for Magnesium is drawn with 4 orbitals. Electron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of electrons like the orbital diagram.
Electronic Configuration Of Elements. In the circuit diagram. A Lewis acid named for the American physical chemist Gilbert N.
Atoms can jump from one orbital to another orbital in the excited state. On 1 May 2014 a paper published in Phys. Relating orbital filling to the Periodic Table.
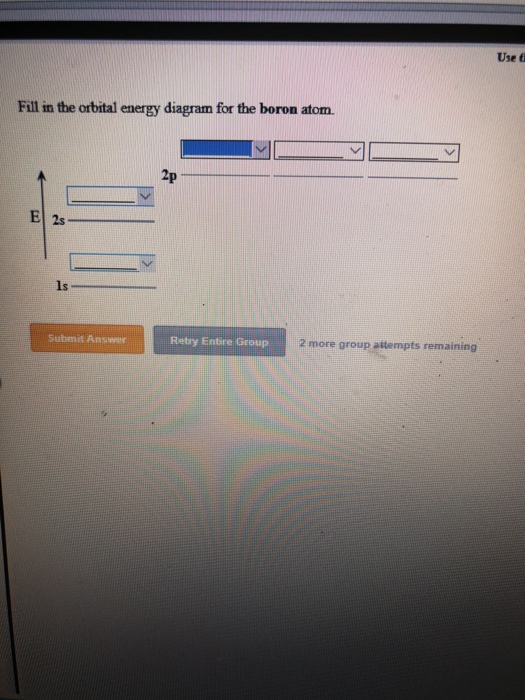
Boron contains 2s 2 2p 1 valence electrons so only one p orbital is needed to. The water cycle also known as the hydrologic cycle or the hydrological cycle is a biogeochemical cycle that describes the continuous movement of water on above and below the surface of the EarthThe mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice fresh water saline water salt water and atmospheric. Note that the ionization energy of boron atomic number 5 is less than that of beryllium atomic number 4 even though the nuclear charge of boron is greater by one proton.
The orbitals are 1s 2s 2p and 3s. Here the boron nitride lattice resolves this challenge by spontaneously breaking. And this diagram shows us the types of orbitals which can be found in the various subshells which are found in the various shells.
Note that the offline orbital rotation is not present in the actual quantum circuit because it is. For light atoms the spinorbit interaction or coupling is small so that the total orbital angular momentum L and total spin S are good quantum numbersThe interaction between L and S is known as LS coupling RussellSaunders coupling named after Henry Norris Russell and Frederick Albert Saunders who described this in 1925 or spin-orbit. On some screens the V for vanadium element 23 may look a bit like a Y.
Is Cs a main group element. A b Energy-level diagram showing the contributions to the interlayer exciton Zeeman shift from the electron spin Δ s black valley Δ v green and atomic orbital Δ a orange for MoSe 2. What is the orbital diagram for Magnesium Mg.
A nuclear thermal rocket NTR is a type of thermal rocket where the heat from a nuclear reaction often nuclear fission replaces the chemical energy of the propellants in a chemical rocketIn an NTR a working fluid usually liquid hydrogen is heated to a high temperature in a nuclear reactor and then expands through a rocket nozzle to create thrust. Division of elements into s p d and f block. The immune system must be actively controlled to prevent inflammatory bowel disease.
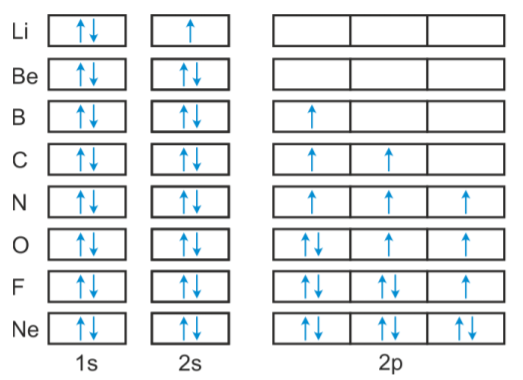
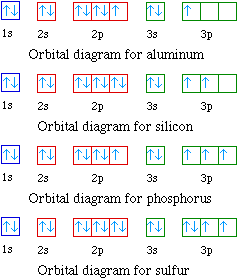
The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5. The atoms boron through neon and hydrogen. Mendeleevs Periodic Law And Table.
This is called quantum jump. The proposed Zeeman-like bias is however an experimental challenge to implement for orbital degrees of freedom. Historical Development Of Periodic Table.
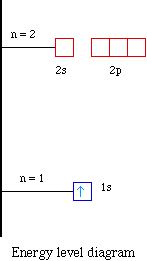
Therefore the electron will first enter the 1s orbital. The p-orbital can have a maximum of six electrons. The short electron configuration of oxygen is 2s 2 2p.
Classification of Elements and Periodicity in Properties. The Magnesium orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital and the remaining two electrons in the 3s orbital. In the above image On the left-hand side the MO diagram of hydronium ion is explained.
11 Uses of Boron Commercial Biological and Miscellaneous. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons. For a diatomic molecule the atomic orbitals of one atom are shown on the left and those of the other atom are shown on the right.
Valence electrons- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic nucleus. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram. Looking at the orbital diagram of oxygen we can see that removing one electron will eliminate the electronelectron repulsion caused by pairing the electrons in the.
Milankovitch cycles describe the collective effects of changes in the Earths movements on its climate over thousands of years. So you have the s subshell the p subshell that has three different orbitals in it you have the d subshell that has one two. Therefore the oxygen full electron configuration will be 1s 2 2s 2 2p 4.
The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil experimentAfter the discovery of the neutron in 1932 models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. And an orbital is a description of that where is it more or less likely to be found. To write the orbital diagram of boronB you have to do the electron configuration of boron.
July 24 2022. Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to. According to Hunds principle the first electron will enter in the.
So the remaining four electrons enter the 2p orbital.
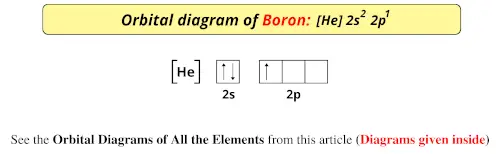
Orbital Diagram Of All Elements Diagrams Given Inside

Quadruple Bonding Between Iron And Boron In The Bfe Co 3 Complex Nature Communications
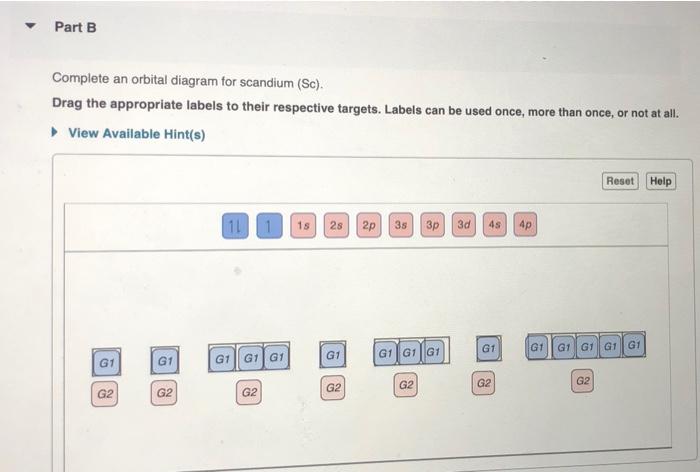
Solved Part A Complete An Orbital Diagram For Boron Drag Chegg Com

Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict Bond Order And Magnetic Properties From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
Fb62ec1c42da2c09faf7d65ed54929a5602a385aec7820 11593015electronicarrangement Png
Chem4kids Com Boron Orbital And Bonding Info
Solved Rule V Part A Complete An Orbital Diagram For Boron Chegg Com

Oneclass Complete An Orbital Diagram For Boron
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration

Fluorine Orbital Diagram Electron Configuration And Valence Electron

Answered Complete An Orbital Diagram For Boron Diagram Chemistry Bar Chart
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration

Molecular Orbital Diagram Wikipedia

Ground State Electron Configurations Of Boron Carbon Nitrogen And Download Scientific Diagram

Honors Chemistry Unit 4 Test Flashcards Quizlet
Solved Use F Fill In The Orbital Energy Diagram For The Chegg Com

Molecular Orbital Diagram For Boron Trifluoride General Chemistry Chemistry Study Guide Chemistry Study Chemistry